|
Learn
and research science, chemistry, biology, physics, math, astronomy,
microscopes, electronics, and much more.
|
|||
|
Learn
and research science, chemistry, biology, physics, math, astronomy,
microscopes, electronics, and much more.
|
|||
Stoichiometry
- COMBINATIONS
OF ELEMENTS AND THEIR REACTIONS:
Study chemical reactions by reading  sections
on stoichiometry in chemistry text books and demonstrating them with laboratory
experiments. (See laboratory cautions below). Combining elements to form
new combinations is a very important part of chemistry. (If you don't know the
meaning of the words here look them up in the "TERMS" links provided
below. Also look up the words in your dictionary for additional
understanding.) Learn about the concept of a
MOLE. Learn more about chemical reactions HERE.
sections
on stoichiometry in chemistry text books and demonstrating them with laboratory
experiments. (See laboratory cautions below). Combining elements to form
new combinations is a very important part of chemistry. (If you don't know the
meaning of the words here look them up in the "TERMS" links provided
below. Also look up the words in your dictionary for additional
understanding.) Learn about the concept of a
MOLE. Learn more about chemical reactions HERE.
In
a simple equation, such as the synthesis of iron(II) sulfide,
iron + sulfur = iron(II) sulfide the equation requires no special balancing:
Fe + S Ž FeS
Our last stage is to put in state symbols, (s, l, g, aq), as appropriate.
Fe(s) + S(s) Ž FeS(s)
On a molar basis, we can say that one mole of iron (56g) reacts with one mole of sulfur (32g) to produce one mole of the compound iron(II) sulfide (88g).
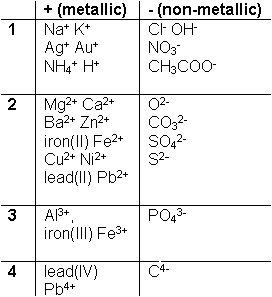 You
will need the ability in chemistry to describe formulas for any substance you
come across. For ionic compounds the compound formula may be arrived at by
knowing the number of charges each ion has. Memorize this table. For
example sodium (Na) has a charge of +1. Oxygen (O) has a charge of -2.
Carbon (C) has a charge of -4. When you see these elements in a formula;
substitute the charge numbers and that will tell you how many atoms of each
element are needed to balance the equation.
You
will need the ability in chemistry to describe formulas for any substance you
come across. For ionic compounds the compound formula may be arrived at by
knowing the number of charges each ion has. Memorize this table. For
example sodium (Na) has a charge of +1. Oxygen (O) has a charge of -2.
Carbon (C) has a charge of -4. When you see these elements in a formula;
substitute the charge numbers and that will tell you how many atoms of each
element are needed to balance the equation.
For example if you combine sodium (Na) and chlorine (Cl) that is the same as +1 = -1 = 0 so you only need one of each. When we say one, it could mean one atom or one mole. So the formula for a combination of sodium and chlorine is Na+Cl- or often written with out the superscripts as NaCl. This is sodium chloride or common table salt. What about aluminum and chlorine? +3 = -1 = +2 In this case you need two chlorine (whatever) to balance the equation for the compound. It would end up as Al3+ 3 Cl- which is AlCl3. Some elements can have various charges like iron. They are written with roman numerals to indicate the charge; iron(II) Fe2+, iron(III) Fe3+, etc.
Stoichiometry - CHEMICAL REACTIONS
Reactions
http://web.jjay.cuny.edu/~acarpi/NSC/6-react.htm
Graphics
http://ull.chemistry.uakron.edu/genobc/Chapter_05/
Key Points http://www.chem4kids.com/files/react_intro.html
Common types of reactions http://www.towson.edu/~ladon/react.html
Predicting reactions http://www.spusd.k12.ca.us/chemmybear/preactions.html
Chemical Reactions http://www.visionlearning.com/library/module_viewer.php?mid=54
Chemistry
Experiments you can do at home - http://www.bxscience.edu/~chinyu/2690/exper/exper.htm
Lab Books
Online - Phoenix College http://chemlab.pc.maricopa.edu/labbooks/default.html
Lucid
stoichiometry (LSU)
Hints
for solving stoichiometry problems (Widener U)
Stoichiometry:
mass-mass problems (ChemCentral)
Stoichiometry
worksheet (Georgetown College)
Stoichiometry
introduction (limiting reagent) (Carnegie Mellon)
Stoichiometry
(limiting reagent simulation)
Stoichiometry
1 (Wilton High School)
Stoichiometry 2 (Wilton High School)
Stoichiometry
3 (Wilton High School)
Stoichiometry
4 (Wilton High School)
Stoichiometry
(DBHS)
Stoichiometry
by the recipe (John Brodemus)
Stoichiometry
applet (IrYdium Project)*
Stoichiometry
notes (Erik)
What
is stoichiometry (Floyd College)
Chemical
reaction stoichiometry web site (Smith)
Mass
relationships in chemical equations (CSU-DH)
Stoichiometry
online (GSU)
BioChemisty/MicroChemistry
Software Downloads.
Good Chemistry
Tutorial Site
High School Chemistry Tutorials Complete http://enc.org/weblinks/science/0,1578,1%2DChemistry,00.shtm